Flow cytometry is a method for simultaneously analyzing multiple physical properties of an individual cell as it flows through a beam of light in a fluid stream, including the cells size and fluorescence. In practice, flow cytometry is essentially a combination of particle counting and fluorescence spectroscopy. Since we have written about both of these subjects in depth in the past, this blog will not spend very much time on the fundamentals of these two technologies instead you are recommended to read our previous blog post on Optical Particle Counting and our white paper on Multi-Color Fluorescence. By combining these two techniques flow cytometry can be employed in cell counting, cell sorting, biomarker detection, and protein engineering analyzing thousands of cells per second as they pass through the liquid stream. In this blog post, we will explore the differences between traditional particle counting instrumentation and those used in flow cytometry. Furthermore, we will discuss how these differences affect the choice of laser or multiple lasers for the particular application, and how this adds additional complexity to the system. It is important to note that this blog post is not meant to serve as an overview of the applications of flow cytometry but rather the fundamental practicalities required for successful laser selection and implementation.
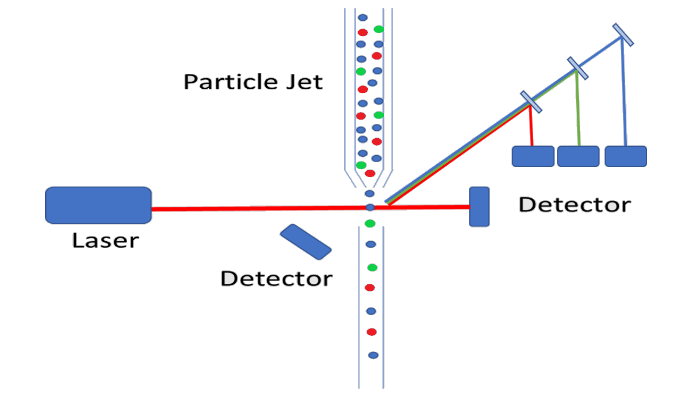
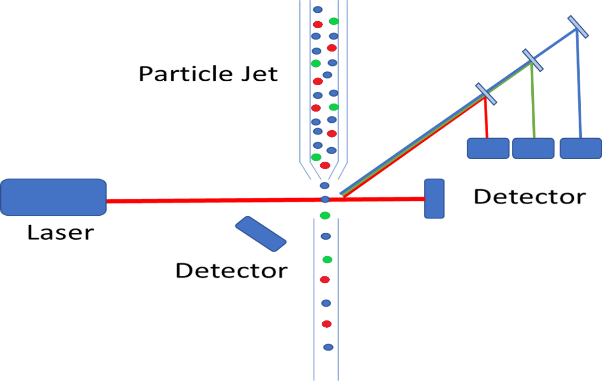
On a fundamental level, the basic instrumentation set-up is very similar to that of traditional particle counting devices, where a particle jet is used to direct a stream of individual particles through the laser path. Where the complexity arises is that we are no longer merely measuring the intensity of scattered light, but we are also required to measure the wavelength of the fluorescence emission. As we discussed in our previous white paper on the subject, fluorophores can be functionalized and bound to living cells. This provides the ability to identify particular cells which fluorophores of varying emission bands, to sort cells based on a wide variety of criteria. Typically, these bands are relatively broad and fairly spread out from each other eliminating the need for costly spectrometers, but the emission path must still be filtered, and dichroicly separated. This allows for each individual emission band to be detected using a single (or double for redundancy purposes) photodetector. The figure below shows a schematic representation of how a particle counter could be modified to measure fluorescence from tagged cells. In this diagram, the different cells are color-coded red, green, and blue, and the fluorescence emission is collected off axis to minimize excitation laser bleed-through.
The Matchbox series offers excellent performance and reliability in an ultra-compact “all-in-one” integrated laser head. They come standard with an integrated internal voltage up-conversion that allows using a 5V power supply while maintaining low noise operation. The monolithic design of the Matchbox Series laser includes thermally stabilized optics in a hermetically sealed housing, ensuring reliable and maintenance-free operation. All the Matchbox series modules include a 12-month warranty and are RoHS compliant. All of these features make them the ideal laser source for integration into commercial flow cytometers.
For detailed technical specifications on the full range of ultra-compact single-mode lasers, click here.
Talk to one of our knowledgeable Product Managers today by emailing us at [email protected] or Contact Us with the button below!
Have questions?

 BUY NOW
BUY NOW